INTRODUCTION:
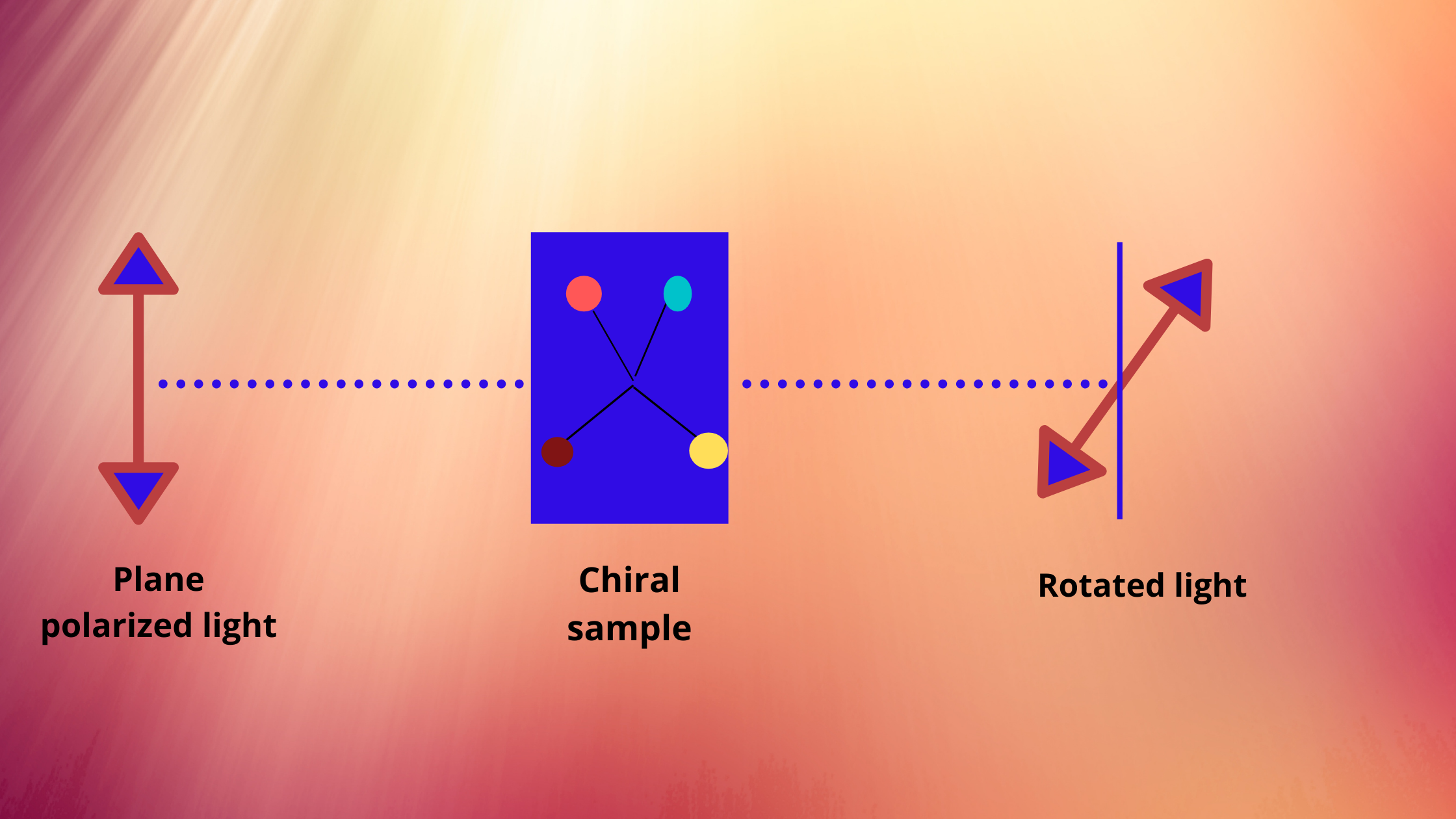
Any material that rotates the plane of polarised light is optically active. Optically active compounds are non-superimposable on their mirror image. This property of non-superimposability of an object on its mirror image is called chirality.
In each case of optical activity of a compound, there are two isomers called enantiomers, which differ in structure only in left and right-handedness of their structure. They rotate the plane of polarised light in opposite directions, although in equal amounts.
The isomer that rotates the plane to the left is called the levo isomer and is designated (-), while the one that rotates the plane to the right is called dextro and is designated as (+).
THE CAUSE OF OPTICAL ACTIVITY/ ROTATION OF THE PLANE

- When light interacts with any molecule, it is slowed down. The extent of interaction depends on the polarizability of the material.
- Plane polarised light can be considered as made up of two circularly polarised light.
- Circularly polarised light can be imagined as helix propagating around the axis of light motion, and one kind is left- and the other is a right-handed helix.
- When the plane polarised light passes through a symmetrical region, the two circularly polarized components travel at the same speed.
- A chiral molecule has different polarizability depending on whether it is approached from the right or left.
- If one circularly polarized component approaches the molecule to say left, it sees different polarizability( hence on a net scale, a different refractive index) than the other and is slowed down to a different extent.
- This would seem to mean that the two circularly polarised light travel at different however it is impossible for two components of the same light to travel at different velocities.
- The “faster” component seems to pull the other component towards it, resulting in rotation of the plane.
SOURCE- JERRY MARCH ADVANCED ORGANIC CHEMISTRY(SIXTH EDITION)